Kinetics of the defunctionalization of oxidized few-layer graphene nanoflakes†
Abstract
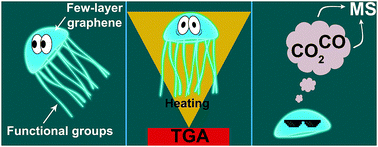
Thermal defunctionalization of oxidized jellyfish-like few-layer graphene nanoflakes was studied under non-isothermal conditions by simultaneous thermal analysis. Activation energies for thermal decomposition of different oxygen functional groups were calculated by the Kissinger method and compared with those for oxidized carbon nanotubes. Oxygen content in graphene nanoflakes was found to significantly affect the decomposition activation energies of carboxylic and keto/hydroxy acids because of their acceptor properties and strong distortion of the graphene layers at the edges of the nanoflakes. The structure of the carbon material and the oxygen chemical state significantly influence the decomposition kinetics of thermally stable oxygen-containing groups. The activation energy for thermal decomposition of phenol groups (110–150 kJ mol−1) is close to that for graphene oxide reduction.


 Please wait while we load your content...
Please wait while we load your content...