Modeling of the electrochemical double layer and its impact on intercalation reactions
Abstract
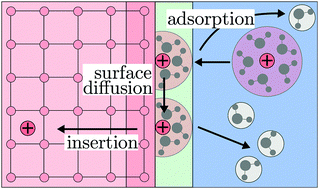
We deduce a generic interface theory to describe charge and electron transfer reactions at electrified interfaces based on fundamental principles. Considering the contact between a solid electrode and a liquid electrolyte, the model allows the consistent determination of emerging electric fields and space charge layers at the interface. The interaction between charge transport and the electrochemical double layer at the boundary between the electrode and electrolyte is investigated. Time scales of interfacial reaction and transport processes are analyzed. Further, we present a reduced bulk model of the electrochemical interface, which allows us to incorporate properties of the electrochemical double layer as extended boundary conditions in transport models of lithium-ion batteries. Numerically less intensive, the reduced model builds a bridge between our detailed description of reactions at electrified interfaces and commonly used modeling approaches for electrode reactions. With both the full and the reduced model, we simulate the impedance response of an electrochemical cell.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...