First-principle atomistic thermodynamic study on the early-stage corrosion of NiCr alloy under fluoride salt environment†
Abstract
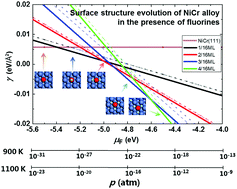
The atomic morphology change in the NiCr alloy surface induced by fluorine-chemisorption was investigated by the ab initio atomistic thermodynamic method to elucidate early-stage corrosion processes of nickel-based alloys in strong oxidizing environment. The surface phase diagrams of Cr-doped Ni(111) surface as a function of fluorine chemical potential were obtained to track the surface structures that are most likely to be fostered in various temperature and pressure conditions. The adsorption of fluorine on the top site of Cr in the alloy surface was the most energetically favorable one. With increasing fluorine chemical potential, more fluorine atoms started to agglomerate in the trapping sink of Cr. Fluorine–fluorine repulsion interaction coupled with strong F–Cr bonding could facilitate a decided morphology modification of the metal substrate. Moreover, an insight into the desorption pathways for potential species revealed that in the presence of fluorine, the dissociation of Cr predominantly stems from the relatively easy desorption in the form of CrF2/CrF3 molecules from the non-passivated Ni-based alloy surface.


 Please wait while we load your content...
Please wait while we load your content...