Lysine residues control the conformational dynamics of beta 2-glycoprotein I†
Abstract
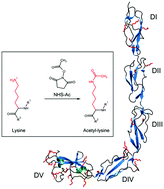
One of the major problems in the study of the dynamics of proteins is the visualization of changing conformations that are important for processes ranging from enzyme catalysis to signaling. A protein exhibiting conformational dynamics is the soluble blood protein beta 2-glycoprotein I (beta2GPI), which exists in two conformations: the closed (circular) form and the open (linear) form. It is hypothesized that an increased proportion of the open conformation leads to the autoimmune disease antiphospholipid syndrome (APS). A characteristic feature of beta2GPI is the high content of lysine residues. However, the potential role of lysine in the conformational dynamics of beta2GPI has been poorly investigated. Here, we report on a strategy to permanently open up the closed protein conformation by chemical acetylation of lysine residues using acetic acid N-hydroxysuccinimide ester (NHS-Ac). Specific and complete acetylation was demonstrated by the quantification of primary amino groups with fluoraldehyde o-phthalaldehyde (OPA) reagent, as well as western blot analysis with an anti-acetylated lysine antibody. Our results demonstrate that acetylated beta2GPI preserves its secondary and tertiary structures, as shown by circular dichroism spectroscopy. We found that after lysine acetylation, the majority of proteins are in the open conformation as revealed by atomic force microscopy high-resolution images. Using this strategy, we proved that the electrostatic interaction of lysine residues plays a major role in stabilizing the beta2GPI closed conformation, as confirmed by lysine charge distribution calculations. We foresee that our approach will be applied to other lysine-rich proteins (e.g. histones) undergoing conformational transitions. For instance, conformational dynamics can be triggered by environmental conditions (e.g. pH, ion concentration, post-translational modifications, and binding of ligands). Therefore, our study may be relevant for investigating the equilibrium of protein conformations causing diseases.


 Please wait while we load your content...
Please wait while we load your content...