Hydrogen detachment driven by a repulsive 1πσ* state – an electron localization function study of 3-amino-1,2,4-triazole†
Abstract
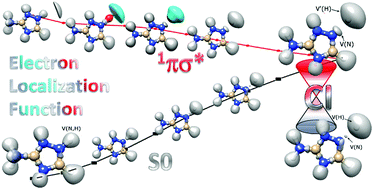
Electron localization function analysis reveals the details of a charge induced hydrogen detachment mechanism of 3-amino-1,2,4-triazole, identified recently to be responsible for phototautomerization of the molecule. In this process vertical excitation to the 1πσ* state is followed by the barrier-less migration of a H atom along the N–H bond toward the conical intersection with the S0 ground state. The most striking feature revealed for the 1πσ* state is partial ejection of σ* electrons outside the molecule, even beyond the NH group, at the Franck–Condon point. Further gradual spatial localization of the electron around the proton moving along the N–H stretching coordinate gives a plausible explanation for the repulsive character of the 1πσ* potential energy surface with the proton wading through the region of space where some negative charge is accumulated (‘a virtual acceptor’), dragging some electron density. This mechanism resembles the one postulated for the hydrogen transfer from a donor molecule (D–H) to an acceptor one (A) in a class of vertically excited molecules with a preexisting inter- or intramolecular D–H⋯A motif, even though the acceptor molecule is absent. The present analysis demonstrates also that the bond evolution and changes in the electron density along the excited state reaction path can be effectively studied with the use of an electron localization function.


 Please wait while we load your content...
Please wait while we load your content...