Cooperative hydrogen bonds form a pseudocycle stabilizing an isolated complex of isocyanic acid with urea†
Abstract
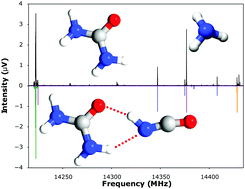
The shapes of macromolecules and their complexes with small molecules are often determined by extended networks of hydrogen bonds. Here, for the first time, we provide a detailed description of a cooperative pair of hydrogen bonds to an individual molecule of urea. The structure and properties of a gas phase complex formed between urea and isocyanic acid are characterised through microwave spectroscopy and ab initio calculations at the CCSD(T)(F12*)/aug-cc-pVTZ level.


 Please wait while we load your content...
Please wait while we load your content...