Phase diagrams of the LiBH4–NaBH4–KBH4 system†
Abstract
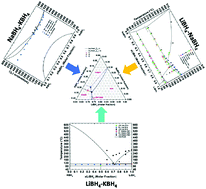
A combination of experimental and computational techniques has been used to fully describe the thermodynamic properties and phase diagrams of the LiBH4–NaBH4–KBH4 system. The Calphad method was used to assess the thermodynamic properties of LiBH4–NaBH4, LiBH4–KBH4, and NaBH4–KBH4 binary systems and to extend the investigation to the LiBH4–NaBH4–KBH4 ternary system. Samples with various compositions in the ternary system were synthesised, both by ball milling and manual mixing of the parent borohydrides, and their thermal stability has been studied using in situ synchrotron radiation X-ray diffraction as a function of temperature and using differential scanning calorimetry. From collected experimental and literature data, a thermodynamic assessment of the ternary system led to the determination of the phase diagrams. In all cases, the solid solutions can be described in the frame of the regular solution model, with interaction parameters positive or equal to zero (i.e. ideal solution). In contrast, the liquid phase was described using negative interaction parameters. A new ternary eutectic composition was estimated and it was confirmed experimentally to be equal to a molar fraction of 0.66LiBH4–0.11NaBH4–0.23KBH4 with a melting temperature of 102 °C.


 Please wait while we load your content...
Please wait while we load your content...