Monolayer germanium monochalcogenides (GeS/GeSe) as cathode catalysts in nonaqueous Li–O2 batteries
Abstract
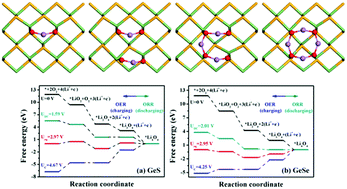
The development of novel cathode catalysts is of great importance to the practical applications of nonaqueous lithium oxygen (Li–O2) batteries. Here by using first-principles calculations, we revealed the catalytic mechanism and evaluated the catalytic activity of monolayer germanium monochalcogenides (2D-GeXs, X = S/Se) as cathode catalytic materials. For 2D-GeXs, Li4O4 with a ring-like structure is the final discharge product. The free energy diagram demonstrates that 2D-GeSe is more energetically favorable than 2D-GeS due to its considerably lower oxygen reduction reaction (ORR) overpotential (0.94 V) and oxygen evolution reaction (OER) overpotential (1.30 V), which originate from its weaker binding with LiO2 and stronger binding with the inserted Li atom. The analyses on electronic properties elucidate that the final product of Li4O4 on 2D-GeSe induces the semiconductor to semi-metal transition. Our results reflect that 2D-GeSe is an excellent candidate as a cathode material in nonaqueous Li–O2 batteries, while 2D-GeS is not appropriate.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...