Insights into the limitations of solar cells sensitized with ruthenium dyes revealed in time-resolved spectroscopy studies†
Abstract
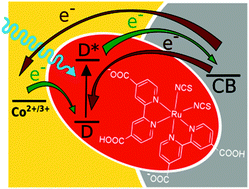
The substitution of iodide electrolytes with cobalt ones has led to the current champion laboratory efficiencies for dye-sensitized solar cells (DSSCs). However, unlike with organic dyes, this strategy does not work with classical ruthenium dyes. Therefore, we compare DSSCs sensitized with a popular Ru dye (N719) using both types of electrolytes by exploring the electron dynamics occurring from sub-ps to seconds. An important limitation in the photocurrent of cobalt-based cells is revealed to be due to electron recombination between titania and oxidized Ru dyes, which is much higher than that in iodide-based cells and occurs on the time scale of tens and hundreds of ps. Electron recombination between titania and the electrolyte, taking place on the millisecond time scale, is responsible for further lowering of the photovoltage and fill factor of cobalt-based cells. Ruthenium dyes also exhibit lower absorption coefficients with respect to their organic counterparts. For this reason, we also investigate the effect of the changes in the titania layer thickness, addition of scattering nanoparticles and modifications in the TiCl4 treatment on DSSC performance.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...