Chemical control of dissolution-driven convection in partially miscible systems: nonlinear simulations and experiments
Abstract
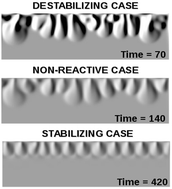
Chemical reactions can impact mixing in partially miscible stratifications by affecting buoyancy-driven convection developing when one phase dissolves into the other one in the gravity field. By means of combined nonlinear simulations and experiments, we explore the power of an A + B → C type of reaction to either enhance or refrain convective dissolution with respect to the nonreactive system depending on the relative contribution to density of the dissolving species A, of the reactant B initially dissolved in the host phase and of the product C. Nonlinear simulations are performed by solving reaction–diffusion–convection equations describing the dissolution and reactive dynamics when a less dense phase of A is layered on top of a reactive denser solution of B, in which A is partially miscible with a given solubility. The spatio-temporal dynamics and convective patterns observed in the numerical study compare favorably with experiments carried out with (i) a liquid alkyl-formate stratified on top of an aqueous solution in which the ester dissolves and undergoes a hydrolysis reaction and (ii) gaseous CO2 dissolving into an aqueous solution of NaOH. We show that the same reaction type can induce a different effect on the convective dynamics depending on the reactant in the host phase. The efficiency of convective dissolution in partially miscible systems can hence be controlled by the chemicals present in the host fluid and their concentration. The direct comparison between the convective dynamics observed during CO2 dissolution in an aqueous phase and in the ester/water stratification validates the latter as a convenient liquid–liquid model system for the interpretation of the impact of chemical reactivity in geological CO2 sequestration.


 Please wait while we load your content...
Please wait while we load your content...