A QM/MM study of the reaction mechanism of human β-ketoacyl reductase†
Abstract
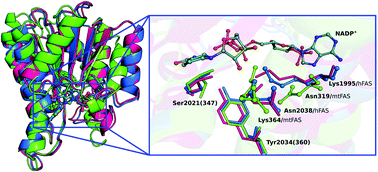
Human fatty acid synthase (hFAS) is a multifunctional enzyme involved in a wide diversity of biological functions. For instance, it is a precursor of phospholipids and other complex processes such as the de novo synthesis of long chain fatty acid. Human FAS is also a component of biological membranes and it is implicated in the overexpression of several types of cancers. In this work, we describe the catalytic mechanism of β-ketoreductase (KR), which is a catalytic domain of the hFAS enzyme that catalyzes the reduction of β-ketoacyl to β-hydroxyacyl with the concomitant oxidation of the NADPH cofactor. The catalysis by KR is an intermediate step in the cycle of reactions that elongate the substrate's carbon chain until the final product is obtained. We study and propose the catalytic mechanism of the KR domain determined using the hybrid QM/MM methodology, at the ONIOM(B3LYP/6-311+G(2d,2p):AMBER) level of theory. The results indicate that the reaction mechanism occurs in two stages: (i) nucleophilic attack by a NADPH hydride to the β-carbon of the substrate, together with an asynchronous deprotonation of the Tyr2034 by the oxygen of the β-alkoxide to hold the final alcohol product; and (ii) an asynchronous deprotonation of the hydroxyl in the NADP+'s ribose by Tyr2034, and of the Lys1995 by the resulting alkoxide in the former ribose to restore the protonation state of Tyr2034. The reduction step occurs with a Gibbs energy barrier of 11.7 kcal mol−1 and a Gibbs reaction energy of −10.6 kcal mol−1. These results have provided an understanding of the catalytic mechanism of the KR hFAS domain, a piece of the heavy hFAS biosynthetic machinery.


 Please wait while we load your content...
Please wait while we load your content...