VUV photochemistry of the H2O⋯CO complex in noble-gas matrices: formation of the OH⋯CO complex and the HOCO radical
Abstract
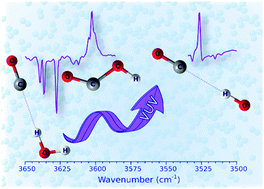
Vacuum ultraviolet (VUV, 130–170 nm) photochemistry of the H2O⋯CO complex is studied by matrix-isolation infrared spectroscopy. The H2O⋯CO complexes in Ne, Ar, Kr, and Xe matrices are generated by ultraviolet (UV, 193 and 250 nm) photolysis of formic acid (HCOOH). VUV photolysis of the H2O⋯CO complexes is found to lead to the formation of the OH⋯CO radical–molecule complexes and trans-HOCO radicals. It is shown that the matrix material, local matrix morphology, and possibly the H2O⋯CO complex geometry strongly affect the VUV photolysis pathways. The intrinsic reactivity of the matrix-isolated OH⋯CO complex resulting in the formation of trans-HOCO is directly demonstrated for the first time. This reaction occurs in Ar, Kr, and Xe matrices upon annealing above 25 K and may proceed over the barrier. The case of a Ne matrix is very special because the formation of trans-HOCO from the OH⋯CO complex is observed even at the lowest experimental temperature (4.5 K), which is in sharp contrast to the other matrices. It follows that quantum tunneling is probably involved in this process in the Ne matrix at such a low temperature. Infrared light also promotes this reaction in the Ne matrix at 4.5 K, which is not the case in the other matrices. The last findings show the effect of the environment on the tunneling and infrared-induced rates of this fundamental chemical reaction.


 Please wait while we load your content...
Please wait while we load your content...