Thermodynamics and kinetics of graphene chemistry: a graphene hydrogenation prototype study†
Abstract
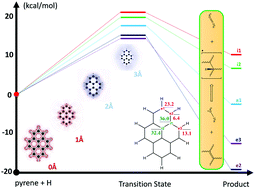
The thermodynamic and kinetic controls of graphene chemistry are studied computationally using a graphene hydrogenation reaction and polyaromatic hydrocarbons to represent the graphene surface. Hydrogen atoms are concertedly chemisorped onto the surface of graphene models of different shapes (i.e., all-zigzag, all-armchair, zigzag-armchair mixed edges) and sizes (i.e., from 16–42 carbon atoms). The second-order Z-averaged perturbation theory (ZAPT2) method combined with Pople double and triple zeta basis sets are used for all calculations. It is found that both the net enthalpy change and the barrier height of graphene hydrogenation at graphene edges are lower than at their interior surfaces. While the thermodynamic product distribution is mainly determined by the remaining π-islands of functionalized graphenes (Phys. Chem. Chem. Phys., 2013, 15, 3725–3735), the kinetics of the reaction is primarily correlated with the localization of the electrostatic potential of the graphene surface.

 Please wait while we load your content...
Please wait while we load your content...