Understanding structural stability of monoclinic LiMnO2 and NaMnO2 upon de-intercalation†
Abstract
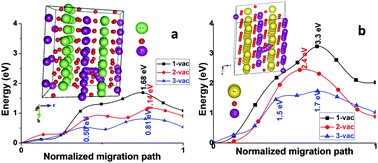
Although many strategies for Li-ion batteries have been successfully transplanted in Na-ion batteries, distinctions between these two kinds of secondary batteries are still clear. For example, monoclinic-NaMnO2 demonstrates high structural stability during charging and discharging, but its iso-structured LiMnO2 transforms to a spinel upon de-lithiation and the specific capacity fades quickly with cycling. In this work, first-principles calculations were carried out to have a better understanding of their difference in structural stability upon de-intercalation. Our studies show that the Mn-ions migrate into the Li layer of LiMnO2via an interstitial tetrahedral O atom when a triple-vacancy of the Li-ion is produced. This process follows a double-vacancy mechanism and results in blocking of the diffusion of other Li-ions. In contrast, it is very difficult for the Mn-ions to migrate into the Na layer in NaMnO2 even when triple-vacancies are generated. The drastic differences between LiMnO2 and NaMnO2 in charge distribution and in the length of the Mn–O bond are believed to be responsible for the Mn-ion migration in them. These findings provide revelations for understanding the de-intercalation behaviors of electrode materials for Li- and Na-ion batteries as well as insights into the structural stability of LiMnO2vs. NaMnO2 upon alkali metal ion de-intercalation.

 Please wait while we load your content...
Please wait while we load your content...