Is the chemistry of lawrencium peculiar?†
Abstract
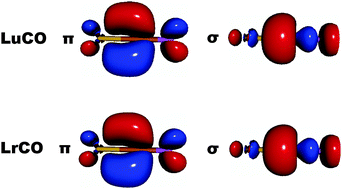
It is explicitly verified that the atomic 7p1 ground-state configuration of Lr originates from relativistic effects. Without relativity one has 6d1. All three ionization potentials IP1–3 of Lr resemble those of Lu. Simple model studies on mono- and trihydrides, monocarbonyls or trichlorides suggest no major chemical differences between Lr and the lanthanides.

 Please wait while we load your content...
Please wait while we load your content...