Investigations on HONO formation from photolysis of adsorbed HNO3 on quartz glass surfaces
Abstract
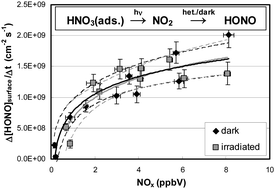
During the last few decades, nitrous acid (HONO) has attracted significant attention as a major source of the OH radical, the detergent of the atmosphere. However, the different daytime sources identified in the laboratory are still the subject of controversial discussion. In the present study, one of these postulated HONO sources, the heterogeneous photolysis of nitric acid (HNO3), was studied on quartz glass surfaces in a photo flow-reactor under atmospherically relevant conditions. In contrast to other investigations, a very low HNO3 photolysis frequency for HONO formation of J(HNO3 → HONO) = 2.4 × 10−7 s−1 (0° SZA, 50% r.h.) was determined. If these results can be translated to atmospheric surfaces, HNO3 photolysis cannot explain the significant HONO levels in the daytime atmosphere. In addition, it is demonstrated that even the small measured yields of HONO did not result from the direct photolysis of HNO3 but rather from the consecutive heterogeneous conversion of the primary photolysis product NO2 on the humid surfaces. The secondary NO2 conversion was not photo-enhanced on pure quartz glass surfaces in good agreement with former studies. A photolysis frequency for the primary reaction product NO2 of J(HNO3 → NO2) = 1.1 × 10−6 s−1 has been calculated (0° SZA, 50% r.h.), which indicates that renoxification by photolysis of adsorbed HNO3 on non-reactive surfaces is also a minor process in the atmosphere.
- This article is part of the themed collection: 2016 most accessed PCCP articles

 Please wait while we load your content...
Please wait while we load your content...