Unraveling the interplay between hydrogen bonding and rotational energy barrier to fine-tune the properties of triazine molecular glasses†
Abstract
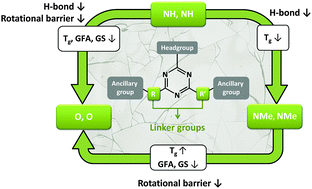
Mexylaminotriazine derivatives form molecular glasses with outstanding glass-forming ability (GFA), high resistance to crystallization (glass kinetic stability, GS), and a glass transition temperature (Tg) above room temperature that can be conveniently modulated by selection of the headgroup and ancillary groups. A common feature of all these compounds is their secondary amino linkers, suggesting that they play a critical role in their GFA and GS for reasons that remain unclear because they can simultaneously form hydrogen (H) bonds and lead to a high interconversion energy barrier between different rotamers. To investigate independently and better control the influence of H bonding capability and rotational energy barrier on Tg, GFA and GS, a library of twelve analogous molecules was synthesized with different combinations of NH, NMe and O linkers. Differential scanning calorimetry (DSC) revealed that these compounds form, with a single exception, kinetically stable glasses with Tg values spanning a very broad range from −25 to 94 °C. While variable temperature infrared spectroscopy combined to chemometrics reveals that, on average, around 60% of the NH groups are still H-bonded as high as 40 °C above Tg, critical cooling rates obtained by DSC clearly show that molecules without H-bond donating linkers also present an outstanding GFA, meaning that H bonding plays a dominant role in controlling Tg but is not required to prevent crystallization. It is a high interconversion energy barrier, provoking a distribution of rotamers, that most efficiently promotes both GFA and resistance to crystallization. These new insights pave the way to more efficient glass engineering by extending the possible range of accessible Tg, allowing in particular the preparation of homologous glass-formers with high GS at ambient temperature in either the viscous or vitreous state.

 Please wait while we load your content...
Please wait while we load your content...