Challenging compounds for calculating molecular second hyperpolarizabilities: the triplet state of the trimethylenemethane diradical and two derivatives†
Abstract
The second hyperpolarizability γ of trimethylenemethane (TMM) and two 1,3-dipole derivatives (NXA and OXA) in their triplet ground state has been evaluated at the UCCSD(T) level with the d-aug-cc-pVDZ extended basis set, highlighting that γ decreases from TMM to NXA and OXA, following the opposite order of their permanent dipole moments. These results are then used to benchmark a broad range of levels of approximation. So, the UMP2, UMP4, and UCCSD methods can be used to characterize γ of TMM and NXA but not of OXA. In that case, the large field-induced charge transfer contribution is difficult to handle using the MPn methods and only the UCCSD method provides values close to the UCCSD(T) reference. Turning to the performance of DFT with typical exchange–correlation functionals, the UM06-2X functional, which contains 54% of HF exchange, performs very well with a maximum of 4.5% of difference with respect to the reference values. On the other hand, employing less HF exchange leads to an overestimation of the responses whereas range-separated hybrids generally underestimate the second hyperpolarizabilities. Finally, the use of spin-projected methods for these 1,3-dipole triplet molecules has a little impact since the spin contamination is almost negligible.
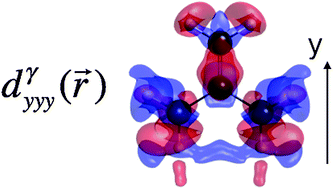

 Please wait while we load your content...
Please wait while we load your content...