Abstract
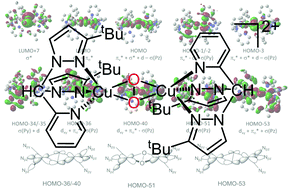
The conformers of the real-life tyrosinase model [Cu2O2{HC(3-tBuPz)2(Py)}2]2+ which displays catalytic hydroxylation reactivity were investigated by density functional theory (DFT) studies including second-order perturbation theory and charge decomposition analysis (CDA). We elucidated the donor competition between pyrazolyl and pyridinyl moieties and found that pyrazolyl units are the stronger donors in bis(pyrazolyl)pyridinylmethane copper complexes. Geometry optimisations and TD-DFT calculations on all conformers proved to be robust in the prediction of the experimental data: the XAS distances and both charge-transfer bands are well reproduced. The CDA analyses gave insights into the electronic structure of the real-life peroxo dicopper species. The donor interplay as well as the multitude of interactions within two prototypical conformers have now been dissected in detail for the first time for a catalytic real-life system without simplifications. We find that the N donor interactions to the core are extremely stabilising and that in the conformer with both pyrazolyl units in equatorial position, these interactions are more stabilising than the axial ones. In the conformer with pyridinyl/pyrazolyl equator, the picture is more mixed but the general trend keeps consistent. We relate the extraordinary catalytic activity of the [Cu2O2{HC(3-tBuPz)2(Py)}2]2+ system to the subtle interplay of the different donor moieties.

 Please wait while we load your content...
Please wait while we load your content...