Solution combustion synthesis of strontium aluminate, SrAl2O4, powders: single-fuel versus fuel-mixture approach
Abstract
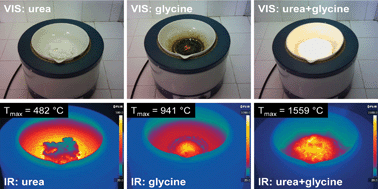
The solution combustion synthesis of strontium aluminate, SrAl2O4, via the classic single-fuel approach and the modern fuel-mixture approach was investigated in relation to the synthesis conditions, powder properties and thermodynamic aspects. The single-fuel approach (urea or glycine) did not yield SrAl2O4 directly from the combustion reaction. The absence of SrAl2O4 was explained by the low amount of energy released during the combustion process, in spite of the highly negative values of the standard enthalpy of reaction and standard Gibbs free energy. In the case of single-fuel recipes, the maximum combustion temperatures measured by thermal imaging (482 °C – urea, 941 °C – glycine) were much lower than the calculated adiabatic temperatures (1864 °C – urea, 2147 °C – glycine). The fuel-mixture approach (urea and glycine) clearly represented a better option, since (α,β)-SrAl2O4 resulted directly from the combustion reaction. The maximum combustion temperature measured in the case of a urea and glycine fuel mixture was the highest one (1559 °C), which was relatively close to the calculated adiabatic temperature (1930 °C). The addition of a small amount of flux, such as H3BO3, enabled the formation of pure α-SrAl2O4 directly from the combustion reaction.

 Please wait while we load your content...
Please wait while we load your content...