Thermodynamics of solvent interaction with the metal–organic framework MOF-5
Abstract
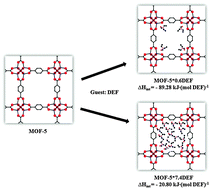
The inclusion of solvent in metal–organic framework (MOF) materials is a highly specific form of guest–host interaction. In this work, the energetics of solvent MOF-5 interactions has been investigated by solution calorimetry in 5 M sodium hydroxide (NaOH) at room temperature. Solution calorimetric measurement of enthalpy of formation (ΔHf) of Zn4O(C8H4O4)3·C3H7NO (MOF-5·DMF) and Zn4O(C8H4O4)3·0.60C5H11NO (MOF-5·0.60DEF) from the dense components zinc oxide (ZnO), 1,4-benzenedicarboxylic acid (H2BDC), N,N-dimethylformamide (DMF) and N,N-diethylformamide (DEF) gives values of 16.69 ± 1.21 and 45.90 ± 1.46 kJ (mol Zn4O)−1, respectively. The enthalpies of interaction (ΔHint) for DMF and DEF with MOF-5 are −82.78 ± 4.84 kJ (mol DMF)−1 and −89.28 ± 3.05 kJ (mol DEF)−1, respectively. These exothermic interaction energies suggest that, at low guest loading, Lewis base solvents interact more strongly with electron accepting Zn4O clusters in the MOF than at high solvent loading. These data provide a quantitative thermodynamic basis to investigate transmetallation and solvent assisted linker exchange (SALE) methods and to synthesize new MOFs.
- This article is part of the themed collection: 2016 most accessed PCCP articles

 Please wait while we load your content...
Please wait while we load your content...