Self-interaction error in DFT-based modelling of ionic liquids†
Abstract
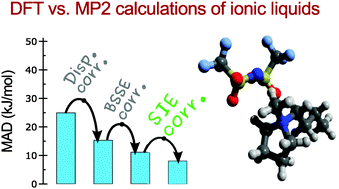
The modern computer simulations of potential green solvents of the future, involving the room temperature ionic liquids, heavily rely on density functional theory (DFT). In order to verify the appropriateness of the common DFT methods, we have investigated the effect of the self-interaction error (SIE) on the results of DFT calculations for 24 ionic pairs and 48 ionic associates. The magnitude of the SIE is up to 40 kJ mol−1 depending on the anion choice. Most strongly the SIE influences the calculation results of ionic associates that contain halide anions. For these associates, the range-separated density functionals suppress the SIE; for other cases, the revPBE density functional with dispersion correction and triple-ζ Slater-type basis is suitable for computationally inexpensive and reasonably accurate DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...