First evidence of the dramatic enhancement of the reactivity of methyl formate (HC(O)OCH3) with OH at temperatures of the interstellar medium: a gas-phase kinetic study between 22 K and 64 K†
Abstract
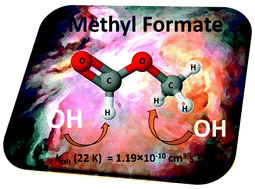
The gas phase chemistry of neutral-neutral reactions of interest in the interstellar medium (ISM) is poorly understood. The rate coefficients (kOH) for the majority of the reactions of hydroxyl (OH) radicals with interstellar oxygenated species are unknown at the temperatures of the ISM. In this study, we present the first determination of kOH for HC(O)OCH3 between 22.4 ± 1.4 and 64.2 ± 1.7 K. The CRESU (French acronym for Cinétique de Réaction en Ecoulement Supersonique Uniforme or Reaction Kinetics in a Uniform Supersonic Flow) technique was used to create a chemical reactor with a uniform temperature and gas density and the pulsed laser photolysis/laser induced fluorescence technique was used to generate OH radicals and to monitor their temporal profile. It was observed that kOH(T) increases by one order of magnitude in only ∼40 K (kOH(T = 22 K) = (1.19 ± 0.36) × 10−10 cm3 s−1 and kOH(T = 64 K) = (1.16 ± 0.12) × 10−11 cm3 s−1) and ∼3 orders of magnitude with respect to kOH(T = 298 K). This reaction is a very efficient route for the loss of HC(O)OCH3 in the gas phase and may have a great impact on the interpretation of astrophysical models of HC(O)OCH3 abundance in the cold regions of the ISM.

 Please wait while we load your content...
Please wait while we load your content...