Singlet oxygen photogeneration from X–O2 van der Waals complexes: double spin-flip vs. charge-transfer mechanism
Abstract
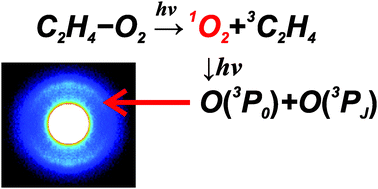
The channel of singlet oxygen O2(1Δg) photogeneration from van der Waals complexes of oxygen X–O2 has been investigated to discriminate between two possible mechanisms based on charge-transfer (CT) or double spin-flip (DSF) transitions. The results obtained in this work for complexes with X = ethylene C2H4, 1,3-butadiene C4H6, deuterated methyl iodide CD3I, benzene C6H6 and water H2O and for those investigated previously indicate the DSF mechanism as a source of singlet oxygen. The formation of O2(1Δg) is observed only when the energy of exciting quantum is sufficient for DSF transition. Universally detected low vibrational excitation of O2(1Δg) arising in the photodissociation of van der Waals complexes X–O2 indicates the DSF mechanism as its source. For complex of ethylene C2H4–O2ab initio calculations of vertical energy ΔEvert for DSF and CT transitions have been carried out. The positive results of singlet oxygen formation from C2H4–O2 can be explained by the DSF but not by the CT mechanism.

 Please wait while we load your content...
Please wait while we load your content...