Thermodynamics of Fe3O4–Co3O4 and Fe3O4–Mn3O4 spinel solid solutions at the bulk and nanoscale
Abstract
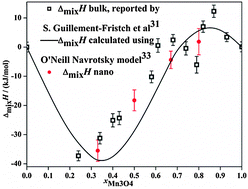
High temperature oxide melt solution calorimetry has been performed to investigate the enthalpies of mixing (ΔmixH) of bulk and nanophase (1 − x)Fe3O4–xM3O4 (M = Co, Mn) spinel solid solutions. The entropies of mixing (ΔmixS) were calculated from the configurational entropies based on cation distributions, and the Gibbs free energies of mixing (ΔmixG) were obtained. The ΔmixH and ΔmixG for the (1 − x)Fe3O4–xCo3O4 system are negative over the complete solid solution range, for both macroscopic and nanoparticulate materials. In (1 − x)Fe3O4–xMn3O4, the formation enthalpies of cubic Fe3O4 (magnetite) and tetragonal Mn3O4 (hausmannite) are negative for Mn3O4 mole fractions less than 0.67 and slightly positive for higher manganese content. Relative to cubic Fe3O4 and cubic Mn3O4 (stable at high temperature), the enthalpies and Gibbs energies of mixing are negative over the entire composition range. A combination of measured mixing enthalpies and reported Gibbs energies in the literature provides experimental entropies of mixing. The experimental entropies of mixing are consistent with those calculated from cation distributions for x > 0.3 but are smaller than those predicted for x < 0.3. This discrepancy may be related to the calculations, having treated Fe2+ and Fe3+ as distinguishable species. The measured surface energies of the (1 − x)Fe3O4–xM3O4 solid solutions are in the range of 0.6–0.9 J m−2, similar to those of many other spinels. Because the surface energies are relatively constant, the thermodynamics of mixing at a given particle size throughout the solid solution can be considered independent of the particular particle size, thus confirming and extending the conclusions of a recent study on iron spinels.

 Please wait while we load your content...
Please wait while we load your content...