The evaporation behavior of sessile droplets from aqueous saline solutions†
Abstract
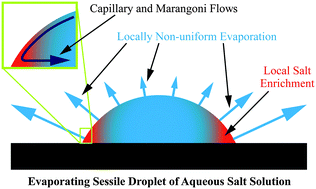
Quantitative experiments on the evaporation from sessile droplets of aqueous saline (NaCl) solutions show a strong dependence on salt concentration and droplet shape. The experiments were performed with seven decades of initial NaCl concentrations, with various droplet sizes and with different contact angles. The evaporation rate is significantly lower for high salt concentrations and small contact angles than what is expected from the well-accepted diffusion-controlled evaporation scenario for sessile droplets, even if the change of the vapor pressure due to the salt is taken into account. Particle tracking velocimetry reveals that this modification of the evaporation behavior is caused by marangoni flows that are induced by surface tension gradients originating from the local evaporative peripheral salt enrichment. In addition it is found that already very low salt concentrations lead to a pinning of the three phase contact line. Whereas droplets with concentration ≥10−6 M NaCl are pinned as soon as evaporation starts, droplets with lower salt concentration do evaporate in a constant contact angle mode. Aside from new, fundamental insights the findings are also relevant for a better understanding of the widespread phenomenon of corrosion initiated by sessile droplets.

 Please wait while we load your content...
Please wait while we load your content...