A computational study of the effect of the metal organic framework environment on the release of chemically stored nitric oxide†
Abstract
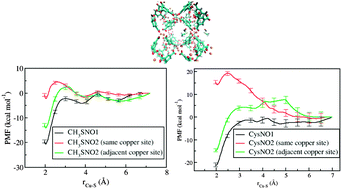
The use of copper based metal organic frameworks as a vehicle for the storage and delivery of chemically stored nitric oxide has been proposed based on recent experiments [J. Am. Chem. Soc., 2012, 134, 3330–3333]. In these experiments copper based metal organic frameworks (MOFs) suspended in ethanol catalytically convert chemically stored nitric oxide (in the S-nitrosothiol or RSNO form) to free nitric oxide at a slow and sustained rate, as compared to a quick release in a solution of ethanol containing free copper ions. In order to gain insight on the effect of the MOF environment on the catalytic activity, a combination of electronic structure calculations on representative clusters and classical simulations using a force-field (partly parameterized on the above calculations) is used to study a simple RSNO species, S-nitrosomethane (CH3SNO) as well as the biologically compatible S-nitrosocysteine, both in the MOF and free copper solution. The free energy profiles of bringing the RSNO species to the catalytic centers have been compared and related to the different solvation environments of the copper catalyst in the complex solvated MOF and in free copper solution. Surprisingly, in the case of the simple CH3SNO moiety as well as the S-nitrosocysteine case, the free energy profile of bringing the first RSNO from the center of one of the pores to the catalytic site in the pore is very similar to the free solution case. On the other hand, bringing a second RSNO molecule to the same catalytic site or to the adjacent catalytic copper site show relatively higher barriers. These studies help shed light on the sustained nitric oxide release in the MOF environment.

 Please wait while we load your content...
Please wait while we load your content...