The atmospheric oxidation mechanism of 2-methylnaphthalene†
Abstract
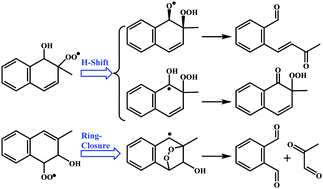
The atmospheric oxidation mechanism of 2-methylnaphthalene (2-MN) initiated by OH radicals is investigated by using quantum chemistry at BH&HLYP/6-311++G(2df,2p) and ROCBS-QB3 levels and kinetic calculations by transient state theory and unimolecular reaction theory coupled with master equation (RRKM-ME). This reaction is mainly initiated by OH additions, forming adducts Rn (2-MN-n-OH, n = 1–8). The fates of R1 and R3, representing the α- and β-adducts, are examined. The fates of R1 and R3 are found to be drastically different. In the atmosphere, R1 reacts with O2via O2 addition to the C2 position to form R1-2OO-a/s, which will undergo a bimolecular reaction with the atmospheric NO or unimolecular isomerization via intramolecular H-shifts, of which the latter is found to be dominant and accounts for the formation of dicarbonyl compounds observed in experimental studies. The role of the tricyclic radical intermediates formed from the ring-closure of R1-2OO is rather limited because their formation is endothermic and reversible, being contrary to the important role of the analogous bicyclic radical intermediates in the oxidation of benzenes. On the other hand, the fate of R3 is similar to that of the benzene–OH adduct, and the tricyclic intermediates will play an important role. An oxidation mechanism is proposed based on the theoretical predictions, and the routes for the experimentally observed products are suggested and compared.

 Please wait while we load your content...
Please wait while we load your content...