Thermodynamics of the hydration equilibrium derived from the luminescence spectra of the solid state for the case of the Eu–EDTA system†
Abstract
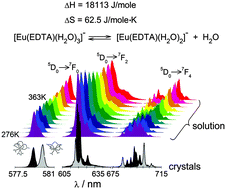
The luminescence properties of two compounds, [C(NH2)3][Eu(EDTA)(H2O)3] (I) and [C(NH2)3]2[Yb0.97Eu0.03(EDTA)(H2O)2]ClO4·6H2O (II), were determined. The weighted sum of luminescence spectra of I and II was used to reproduce the spectra of the Eu–EDTA system in aqueous solution in the temperature range 276–363 K. By implementing this method it was possible to determine the thermodynamic functions (ΔH = 18113 ± 506 J mole−1 and ΔS = 62.5 ± 4.9 J mole−1 K−1) of the reaction [Eu(EDTA)(H2O)3]− ⇆ [Eu(EDTA)(H2O)2]− + H2O, which is difficult using other methods.

 Please wait while we load your content...
Please wait while we load your content...