Adsorption studies of divalent, dinuclear coordination complexes as molecular spacers on SWCNTs†
Abstract
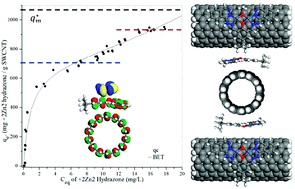
In order to enhance the electrical energy storage capabilities of nanostructured carbon materials, inter-particle spacer strategies are needed to maintain ion-accessible surface area between the nanoparticles. This paper presents a comparison between different classes of divalent, dinuclear coordination complexes which both show strong adsorption to SWCNTs and have molecular spacer properties that maintain electrochemical activity. We find that a novel, dinuclear zinc hydrazone complex binds as an ion-pair at very high loading while not inducing significant aggregation as compared to our previously studies of dinuclear ruthenium complexes. These conclusions are supported by conductivity and dispersion stability data. Moreover, since zinc is an earth abundant metal, these complexes can be used as components in sustainable energy storage materials. Binding kinetics and binding equilibrium data are presented. Modeling of the adsorption isotherm is best fit with the BET model. Kinetics data support an independent binding model. Preliminary capacitance and membrane resistance data are consistent with the complexes acting as molecular spacers between the SWCNTs in a condensed thin film.

 Please wait while we load your content...
Please wait while we load your content...