The reactivity of stoichiometric tungsten oxide clusters towards carbon monoxide: the effects of cluster sizes and charge states†
Abstract
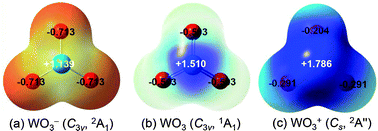
Density functional theory (DFT) calculations are employed to investigate the reactivity of tungsten oxide clusters towards carbon monoxide. Extensive structural searches show that all the ground-state structures of (WO3)n+ (n = 1–4) contain an oxygen radical center with a lengthened W–O bond which is highly active in the oxidation of carbon monoxide. Energy profiles are calculated to determine the reaction mechanisms and evaluate the effect of cluster sizes. The monomer WO3+ has the highest reactivity among the stoichiometric clusters of different sizes (WO3)n+ (n = 1–4). The reaction mechanisms for CO with mono-nuclear stoichiometric tungsten oxide clusters with different charges (WO3−/0/+) are also studied to clarify the influence of charge states. Our calculated results show that the ability to oxidize CO gets weaker from WO3+ to WO3− as the negative charge accumulates progressively.

 Please wait while we load your content...
Please wait while we load your content...