Efficient size control of copper nanoparticles generated in irradiated aqueous solutions of star-shaped polyelectrolyte containers
Abstract
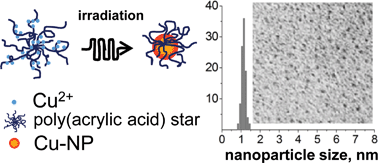
The formation of copper nanoparticles (Cu-NPs) in irradiated aqueous solutions of star-shaped poly(acrylic acid) (PAA) were studied at two pH values. Transmission electron microscopy (TEM) demonstrates that the star-shaped macromolecules loaded with Cu2+ ions can act as individual nanosized containers providing a perfect control over the size and size distribution of Cu-NPs. Electron paramagnetic resonance (EPR) and optical spectroscopy show a transformation of mechanisms controlling the reduction of Cu2+ ions and the further formation of Cu-NPs. At pH 2.9, Cu-NPs are formed from the aquacomplexes of Cu2+ ions through homogeneous nucleation. At pH 4.3, the formation of Cu-NPs occurs inside macromolecular containers loaded with Cu2+ ions, which are bound to carboxylic groups of the polyelectrolyte. In the latter case, Cu-NPs apparently ripen from preformed hydrated Cu2O seeds, which are thought to result from the ultrasmall (Cu2+)m(OH−)k(COO−)n species, thus implying a heterogeneous nucleation.

 Please wait while we load your content...
Please wait while we load your content...