The synergistic mechanism of graphene and MoS2 for hydrogen generation: insights from density functional theory
Abstract
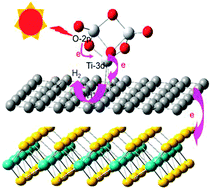
The synergistic effect of graphene and MoS2 was investigated by using density functional theory (DFT) calculations on the enhanced photocatalytic H2 production activity of TiO2/graphene/MoS2 ternary nanoparticles. Our results indicate that it can form a weak covalent bond between the Ti atom of TiO2 nanocluster and the nearest C atom on graphene, which not only makes the original degenerate C(2p) orbital level of the graphene (part of the conduction band energy level) split, resulting in the production of a lower level of C(2p) that makes it easier to accept the excited electron from the Ti(3d) orbital, but also forms a +/− sequence electric field in the interface between them. It is conclusive that the electron moves from the TiO2 cluster to the graphene. In addition, we also find that the band gap of the TiO2 cluster can be doped by the graphene and MoS2, and the conduction band consists predominantly of C(2p), S(3p) and Mo(4d) orbital energy level near the Fermi level. These results illustrate that the excited electron will eventually accumulate in the graphene or MoS2 film, which can effectively enhance the separation between the excited electrons and the holes in the TiO2 clusters, thereby increasing the efficiency of hydrogen evolution. Our results are consistent with the experimental results, and can provide some valuable information for the design of photocatalytic composites.

 Please wait while we load your content...
Please wait while we load your content...