Activation enthalpies and entropies of the atropisomerization of substituted butyl-bridged biphenyls
Abstract
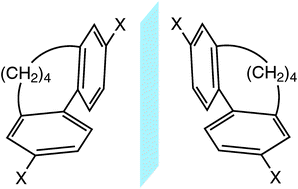
A combined quantum chemical and experimental study of the atropisomerization energies of di-para-substituted butyl-bridged biphenyl cyclophanes is presented. We studied the influence of electron donor and electron acceptor substituents on the height of the enantiomerization barrier and examined the enthalpic and entropic contributions. The reaction pathway with minimum and transition state structures was established using density functional theory calculations. The Gibbs free activation energies derived from this pathway correspond well to the ones determined by temperature dependent high performance liquid chromatography (HPLC) measurements. Surprisingly, large discrepancies were found for the contributions of enthalpy and entropy. Thermodynamic data derived from circular dichroism (CD) measurements support the quantum chemical calculations for the distribution of enthalpy and entropy, contrary to the HPLC measurements. Rationalizations for this are given.

 Please wait while we load your content...
Please wait while we load your content...