Outside rules inside: the role of electron-active substituents in thiophene-based heterophenoquinones†
Abstract
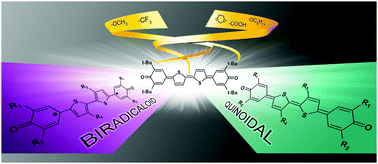
The biradicaloid vs. quinoidal character of the ground state of thiophene-based heterophenoquinones bearing donor or acceptor groups is investigated. Keeping the conjugation length fixed, namely, the 5,5′-bis(3,5-di-tert-butyl-4-oxo-2,5-cyclohexadiene-1-ylidene)-2,2′-dihydroxy bithiophene backbone, an opposite effect occurs depending on the donating or withdrawing nature of the substituents. The character of the ground state depends not only on the electronic nature of the substituents but also on their position on the molecular skeleton: donor groups on the 3,3′-positions of the bithiophene central core stabilize a quinoidal ground state, whereas a biradicaloid electronic structure results from the introduction of the same donor groups onto the lateral phenones. Withdrawing groups behave similar to donors, but in the opposite direction.

 Please wait while we load your content...
Please wait while we load your content...