Voltammetric study of the boric acid–salicylaldehyde–H-acid ternary system and its application to the voltammetric determination of boron†
Abstract
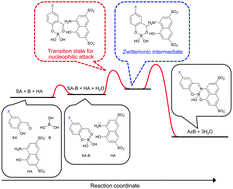
The ternary system of boric acid, salicylaldehyde (SA) and H-acid (HA) was voltammetrically studied from kinetic and equilibrium points of view. The effect of the SA substituents was also studied by using two analogs, 5-fluorosalicylaldehyde (F-SA) and 5-methylsalicylaldehyde (Me-SA). The three cathodic peaks of Azomethine H (AzH), Azomethine H–boric acid complex (AzB), and free SA were observed in the solution containing boric acid, SA and HA. The peak potentials of AzH and SA were shifted to negative potentials with increasing pH, while the peak potential of AzB was pH-independent. This difference indicates that a proton participates in the charge-transfer steps of the AzH and SA reductions, but not in that of the AzB reduction. The formation constants for the AzB complexation were similar among all the examined analogs. In the kinetic study, the reaction rate was higher in an acidic condition for the AzH formation, but in a neutral condition for the AzB formation. The rate constants for the AzB complexes were in the order of F-SA > SA ≈ Me-SA, indicating that the fluoro group accelerates the F-AzB complexation. The AzB complexation mechanism is considered to consist of more than three steps, i.e., the pre-equilibrium of the salicylaldehyde–boric acid complex (SA–B) formation, the nucleophilic attack of HA on SA–B, and the remaining some steps to form AzB. Based on these results, the voltammetric determination method of boron using F-SA was optimized, which allowed the boron concentration to be determined within only 5 min with a 0.03 mg B dm−3 detection limit.

 Please wait while we load your content...
Please wait while we load your content...