Mutual solubilities between water and non-aromatic sulfonium-, ammonium- and phosphonium-hydrophobic ionic liquids†
Abstract
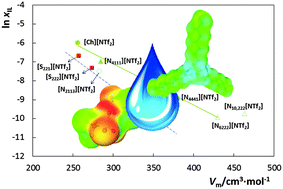
Although previous studies attempted to characterize the liquid–liquid phase behaviour between water and ionic liquids (ILs), the impact of non-cyclic cations on the solubilities is poorly studied and yet to be understood. In this work, the mutual solubilities between water and ILs containing the anion bis(trifluoromethylsulfonyl)imide, [NTf2]−, combined with the cations diethylmethylsulfonium, [S221][NTf2], triethylsulfonium, [S222][NTf2], butyltrimethylammonium, [N4111][NTf2], tributylmethylammonium, [N4441][NTf2], methyltrioctylammonium, [N1888][NTf2], and methyltrioctylphosphonium, [P1888][NTf2], from (288.15 to 318.15) K and at 0.1 MPa, were experimentally measured and further compared with predictions from the COnductor-like Screening MOdel for Real Solvents (COSMO-RS). All the studied phase diagrams display an upper critical solution temperature (UCST). The binary system composed of [P1888][NTf2] exhibits the widest immiscibility gap, followed by [N18888][NTf2], [N4441][NTf2], [S222][NTf2], [N4111][NTf2], and [S221][NTf2]. The COSMO-RS is able to correctly predict the experimental UCST behaviour and the cation impact on the immiscibility regimes observed. Natural Population Analysis (NPA) calculations were additionally performed for the isolated cations in the gas phase indicating that the differences in the water–IL mutual miscibilities might not result only from the hydrophobicity of the cation (derived from the increase of the alkyl chains length) but also from the charge distribution of the central atom and attached methylene groups. This fact explains the enhanced solubility of ammonium-based ILs in water here identified.


 Please wait while we load your content...
Please wait while we load your content...