Buffers more than buffering agent: introducing a new class of stabilizers for the protein BSA†
Abstract
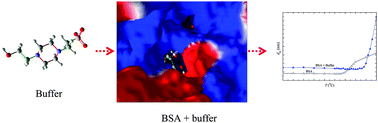
In this study, we have analyzed the influence of four biological buffers on the thermal stability of bovine serum albumin (BSA) using dynamic light scattering (DLS). The investigated buffers include 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES), 4-(2-hydroxyethyl)-1-piperazine-propanesulfonic acid (EPPS), 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid sodium salt (HEPES–Na), and 4-morpholinepropanesulfonic acid sodium salt (MOPS–Na). These buffers behave as a potential stabilizer for the native structure of BSA against thermal denaturation. The stabilization tendency follows the order of MOPS–Na > HEPES–Na > HEPES ≫ EPPS. To obtain an insight into the role of hydration layers and peptide backbone in the stabilization of BSA by these buffers, we have also explored the phase transition of a thermoresponsive polymer, poly(N-isopropylacrylamide (PNIPAM)), a model compound for protein, in aqueous solutions of HEPES, EPPS, HEPES–Na, and MOPS–Na buffers at different concentrations. It was found that the lower critical solution temperatures (LCST) of PNIPAM in the aqueous buffer solutions substantially decrease with increase in buffer concentration. The mechanism of interactions between these buffers and protein BSA was probed by various techniques, including UV-visible, fluorescence, and FTIR. The results of this series of studies reveal that the interactions are mainly governed by the influence of the buffers on the hydration layers surrounding the protein. We have also explored the possible binding sites of BSA with these buffers using a molecular docking technique. Moreover, the activities of an industrially important enzyme α-chymotrypsin (α-CT) in 0.05 M, 0.5 M, and 1.0 M of HEPES, EPPS, HEPES–Na, and MOPS–Na buffer solutions were analyzed at pH = 8.0 and T = 25 °C. Interestingly, the activities of α-CT were found to be enhanced in the aqueous solutions of these investigated buffers. Based upon the Jones–Dole viscosity parameters, the kosmotropic or chaotropic behaviors of the investigated buffers at 25 °C have been examined.

 Please wait while we load your content...
Please wait while we load your content...