A model study on the photochemical isomerization of isothiazoles and thiazoles†
Abstract
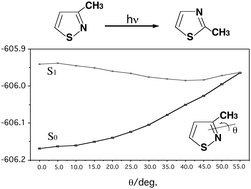
The mechanisms for photochemical isomerization reactions are studied theoretically using three model systems: 3-methylisothiazole, 4-methylisothiazole and 5-methylisothiazole. The CASSCF (ten-electron/seven-orbital active space) and MP2-CAS methods are used with the 6-311G(d) and 6-311++G(3df,3pd) basis sets, respectively. Three mechanisms, the internal cyclization–isomerization route (path A), the ring contraction–ring expansion route (path B) and the direct route (path C), are used to determine the actual photochemical reaction mechanism for these three model molecules. The structures of the conical intersections, which play a crucial role in these photo-transpositions, are determined. The intermediates and transition structures of the ground states are also calculated, to give a qualitative explanation of the reaction pathways. These model investigations suggest that the preferred reaction route is: reactant → Franck–Condon region → conical intersection → photoproduct. Particularly, the direct mechanism (path C) described in this work gives a better explanation than other pathways proposed before and is supported by the experimental observations. The results obtained allow a number of predictions to be made.

 Please wait while we load your content...
Please wait while we load your content...