Different aggregation dynamics of benzene–water mixtures
Abstract
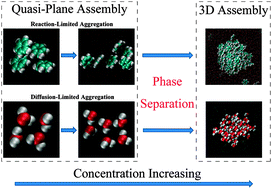
All-atom molecular dynamics simulations for benzene–water mixtures are performed, aiming to explore the relationship between the microscopic structures and the thermodynamic properties, in particular, the transformation dynamics from the mutually soluble state to the phase-separated state. We find that the molecular aggregation of benzene in the water-rich mixture is distinctly different from that of water in the benzene-rich mixture. This aggregation difference is attributed to the different intermolecular interactions: the clustering of benzene molecules in the water-rich mixture is primarily driven by weak short-distance π–π interactions; while the formation of water clusters in the benzene-rich solution is triggered by long-range dipole–dipole electrostatic interactions. Moreover, the molecular aggregations show double-scaled features: firstly assembling in a quasi-plane at a low concentration, then bulking in three dimensions with an increase in concentration.

 Please wait while we load your content...
Please wait while we load your content...