Gas storage potential of ExBox4+ and its Li-decorated derivative†
Abstract
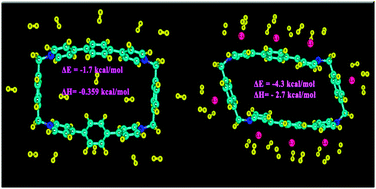
The newly synthesized compound ExBox4+ is investigated for possible gas storage ability. The presence of high charge as well as aromatic rings which are some of the anticipated criteria for favorable gas storage makes ExBox4+ a promising compound for gas adsorption. Considering the two important problems being faced by humankind, viz., energy and environment, possible adsorption of H2 and CO respectively on recently synthesized compound ExBox4+ is studied with the help of density functional theory (DFT). Endohedral hydrogen molecules interact more strongly than exohedral molecules. The hydrogen storage capacity appears to be ∼4.3 wt% which is close to that of some recently studied materials like ZIF and PAF. The endohedral CO sorption is also analyzed with the help of DFT. The dynamics of the gas bound compound are studied with the help of ADMP simulation. The effects of counter ions on the adsorption energy and bonding interaction are also investigated. The first principle DFT calculation and MD simulation are performed to investigate the effect of lithium doping on the gas adsorbing capacity and adsorption enthalpy as well as adsorption energy of ExBox4+. The Li decorated ExBox4+ is capable of adsorbing 6.23 wt% of hydrogen which almost satisfies the goal of DOE. Adsorption of CO on the metal decorated ExBox4+ is also studied. The nature of the bonding interaction between the gas molecule and the adsorbent is studied with the help of AIM and EDA as well as charge decomposition analysis is performed.

 Please wait while we load your content...
Please wait while we load your content...