New ab initio potential energy surfaces for the ro-vibrational excitation of OH(X2Π) by He
Abstract
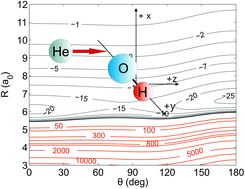
We present a new set of three-dimensional potential energy surfaces (PES) for the OH(X2Π)–He van der Waals system, which explicitly takes into account the OH vibrational motion. Ab initio calculations of the OH–He PES were carried out using the open-shell single- and double-excitation coupled cluster approach with non-iterative perturbational treatment of triple excitations [RCCSD(T)]. The augmented correlation-consistent aug-cc-pVXZ (X = Q, 5, 6) basis sets were employed, and the energies obtained were then extrapolated to the complete basis set (CBS) limit. Integral and differential cross sections (ICS and DCS), and thermal rate coefficients for the rotational excitation in OH + He collisions were calculated using the new PES, and compared with available experimental results. Experimental and theoretical results were found to be in a very good agreement. The newly constructed PES reproduces the available experimental results for OH(X2Π, v = 0, 1) + He collisions better than the previously available two-dimensional PESs, which were constructed using a fixed OH bond distance. Our work provides the first RCCSD(T) PES for future anticipated experiments in OH(X2Π, v ≥ 0) + He collisions.

 Please wait while we load your content...
Please wait while we load your content...