The effect of nitrogen doping on mercury oxidation/chemical adsorption on the CuCo2O4(110) surface: a molecular-level description†
Abstract
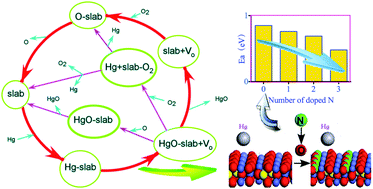
Based on density functional theory (DFT) calculations, the detailed mercury oxidation/chemical adsorption mechanisms on the N-doped CuCo2O4(110) surface are studied. The DFT calculations show that Ow (bonded with one Cu2+ ion and one Co3+ ion) is far more active than Os (bonded with three Co3+ ions) and the mercury oxidation/chemical adsorption activation energy (Ea) on the virgin CuCo2O4(110) surface involving Ow is 0.85 eV. The physically adsorbed mercury overcomes the Ea and enters the energy well that plays an important role in mercury oxidation/chemical adsorption. Nitrogen doping can greatly increase the activity of Ow and decrease the activity of Os at the same time, which greatly affect the mercury oxidation/chemical adsorption abilities on the CuCo2O4(110) surface, and the Ea variation of mercury oxidation/chemical adsorption is as follows: 0.85 eV (virgin CuCo2O4(110)) → 0.76 eV (one N-doped CuCo2O4(110)) → 0.69 eV (two N-doped CuCo2O4(110)) → 0.48 eV (three N-doped CuCo2O4(110)). In addition, N-doping can decrease the adsorption energy of mercury and mercuric oxide. The effect of N-doping on the bonding mechanism of mercury adsorption on the CuCo2O4(110) surface is analyzed by the local density of state (LDOS) and the natural bonding orbital (NBO). The calculation results correspond well to the experimental data.

 Please wait while we load your content...
Please wait while we load your content...