Inherent and transferable stabilization energies of carbon- and heteroatom-centred radicals on the same relative scale and their applications†
Abstract
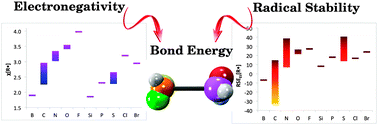
Accurate G3(MP2)-RAD calculations are used to predict 264 R–H, R–CH3, R–Cl and R–R bond dissociation energies for a wide-ranging test set of carbon and non-carbon centred R˙ radicals. The data are used to calculate a set of inherent and transferrable radical stabilization energies, denoted RSEEt, which ranks the inherent stability of the 66 radicals studied on the same relative scale, irrespective of the nature of the radical centre. The Pauling electronegativity parameter for each radical is also calculated from the same data, along with the radical's inherent bonding ability D[R–R]calc. This latter quantity is defined as the R–R bond dissociation energy expected in the absence of direct steric or resonance interactions that are present in R–R but absent in R–CH3 and R–Cl. We show that the differences between D[R–R] and D[R–R]calc are typically very small except when R is sterically bulky, or there is a chain of (hyper)conjugation across the R–R bond. In such cases the difference between D[R–R] and D[R–R]calc provides a convenient means of quantifying the stabilization or destabilization of R–R due to these interactions. The predictability of the scheme is demonstrated by using these radical stabilities to calculate R–R′ bond dissociation energies for 234 combinations of the 66 radicals studied, chosen to exclude steric or resonance interactions in the R–R′ bond. The predicted bond energies lie within an average of 1.6 kcal mol−1 from directly measured or calculated literature values.

 Please wait while we load your content...
Please wait while we load your content...