Network formation in graphene oxide composites with surface grafted PNIPAM chains in aqueous solution characterized by rheological experiments†
Abstract
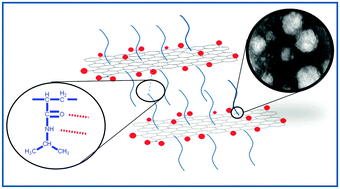
Poly N-isopropyl acrylamide (PNI) radically polymerized in aqueous solution in the presence of graphene oxide (GO) can significantly change the properties of the resulting solution from a regular polymer solution to a soft solid with a GO content of only 0.176 wt% (3 wt% with respect to PNI). However, these properties require the presence of both grafting and supramolecular interactions between polymer chains and hydrophilic groups on GO (–OH, –COOH), proven by Fourier transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), X-ray diffraction and spectroscopy (XRD) and Raman spectra. While very low GO-contents (below 0.05 wt%) only lead to a labile structure, which can be disassembled by shear, higher contents yield composites with solid-like characteristics. This is clearly evident from the rheological behaviour, which changes significantly at a GO content around 0.15 wt%. Intensive shearing destroys the weak network, which cannot reform quickly at lower GO-concentrations, while at intermediate concentrations, restructuring is fast. GO-contents of 0.176 wt% lead to a material behaviour, which almost perfectly recovers from small deformations (creep and creep recovery compliance almost match) but larger deformations lead to permanent damage to the sample.

 Please wait while we load your content...
Please wait while we load your content...