Hydrophobicity alone can not trigger aggregation in protonated mammalian serum albumins
Abstract
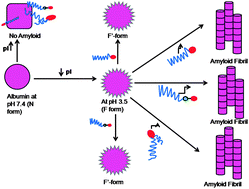
Amyloid fibrils are associated with neurodegenerative disorders and are formed by a number of proteins. In this study, the amyloid-forming behavior of several different serum albumins was examined at pH 3.5 i.e., about two pH units below their isoelectric points (pI ∼ 5.5) to examine the roles played by negative charge and hydrophobicity of exogenously added surfactants such as SDS, SDBS and AOT. The propensities of SDS, SDBS and AOT to promote the formation of amyloid fibrils were investigated by using measurements of turbidity, Rayleigh scattering, ThT and CR dye binding, DLS as well as far-UV CD. At submicellar concentrations of SDS and SDBS (0.5–2.5 mM) amyloid fibrils were formed by all albumins studied whereas at higher concentrations amyloid fibril formation was completely inhibited. Interestingly AOT promoted amyloid fibril formation up to 11 mM without any inhibition. The interaction between the albumins and the surfactants was exothermic, as confirmed by isothermal titration calorimetry (ITC). From the turbidity, Rayleigh scattering and dynamic light scattering data, it was concluded that amyloid induction was promoted most by AOT followed by SDBS and SDS. Similar studies were performed at pH 7.4 i.e., about two units of pH above the albumins pI, and no amyloid fibrils were formed. From these studies we conclude that negatively charged surfactants induce amyloid fibril formation in serum albumins with the help of electrostatic and hydrophobic interactions. Besides the study performed at pH 7.4 indicates that hydrophobic interactions alone can not induce aggregation in serum albumins.

 Please wait while we load your content...
Please wait while we load your content...