Complexes containing CO2 and SO2. Mixed dimers, trimers and tetramers†
Abstract
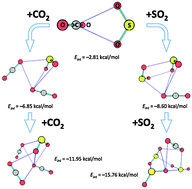
Mixed dimers, trimers and tetramers composed of SO2 and CO2 molecules are examined by ab initio calculations to identify all minimum energy structures. While AIM formalism leads to the idea of a pair of C⋯O bonds in the most stable heterodimer, bound by some 2 kcal mol−1, NBO analysis describes the bonding in terms of charge transfer from O lone pairs of SO2 to the CO π* antibonding orbitals. The second minimum on the surface, just slightly less stable, is described by AIM as containing a single O⋯O chalcogen bond. The NBO picture is that of two transfers in opposite directions: one from a SO2 O lone pair to a π* antibond of CO2, supplemented by CO2 Olp → π*(SO). Decomposition of the interaction energies points to electrostatic attraction and dispersion as the dominant attractive components, in roughly equal measure. The various heterotrimers and tetramers generally retain the dimer structure as a starting point. Cyclic oligomers are favored over linear geometries, with a preference for complexes containing larger numbers of SO2 molecules.

 Please wait while we load your content...
Please wait while we load your content...