Spectroscopic observation of photo-induced metastable linkage isomers of coinage metal (Cu, Ag, Au) sulfur dioxide complexes†
Abstract
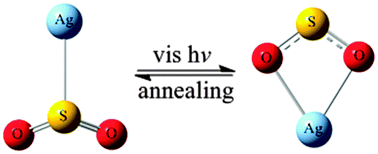
Coinage metal atom (Cu, Ag, Au) reactions with SO2 were investigated by matrix isolation infrared absorption spectroscopy and density functional theory electronic structure calculations. Both mononuclear complexes M(η1-SO2) (M = Ag, Au) and M(η2-O2S) (M = Ag, Cu) were observed during condensation in solid argon or neon. Interestingly, the silver containing mononuclear complexes are interconvertible; that is, visible light induces the isomerization of Ag(η1-SO2) to Ag(η2-O2S) and vice versa on annealing. However, there is no evidence of similar interconvertibility for the Cu(η2-O2S) and Au(η1-SO2) molecules. These different behaviors are discussed within the bonding considerations for all of the obtained products.

 Please wait while we load your content...
Please wait while we load your content...