Enhanced hydrogen sensing properties of graphene by introducing a mono-atom-vacancy
Abstract
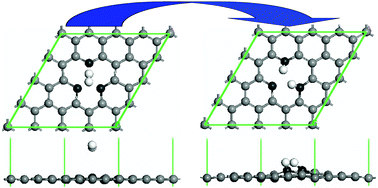
To facilitate the dissociative adsorption of H2 molecules on pristine graphene, the addition of a mono-atom-vacancy to graphene is proposed. This leads to reduction of the dissociative energy barrier for a H2 molecule on graphene from 3.097 to 0.805 eV for the first H2 and 0.869 eV for the second, according to first principles calculations. As a result, two H2 molecules can be easily dissociatively adsorbed on this defected graphene at room temperature. The electronic structure and conductivity of the graphene change significantly after H2 adsorption. In addition, the related dissociative adsorption phase diagrams under different temperatures and partial pressures show that this dissociative adsorption at room temperature is very sensitive (10−35 mol L−1). Therefore, this defected graphene is promising for ultra-sensitive room temperature hydrogen sensing.

 Please wait while we load your content...
Please wait while we load your content...