On the structure of water and chloride ion interactions with a peptide backbone in solution†
Abstract
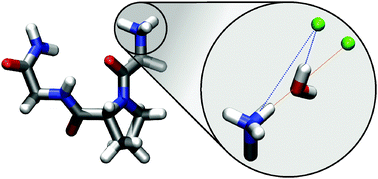
The arrangement of water and chloride ions around a model peptide (glycyl-L-prolyl-glycine-NH2) was investigated using Molecular Dynamics (MD) simulations and complementary Empirical Potential Structure Refinement (EPSR) simulations which adapt the modelled structure to reproduce experimentally measured neutron diffraction data. The results are in good qualitative agreement and show a common picture for all hydrogen-containing amine and amide groups: namely that there are two common chloride interactions observed – a direct contact between Cl− and peptide backbone and a water-mediated interaction. The geometry of this mediation depends on the distance between chloride and nitrogen and hints towards two distinct modes of interaction between water and the ion, either along one of the O–H bonds or along the water dipole.

 Please wait while we load your content...
Please wait while we load your content...